
Consecutive Studies, Continuous Outcome
J. Kyle Wathen and Laurent Spiess
February 19, 2026
ConsecutiveStudiesContinuous.RmdThis example is related to both the Integration Point: Response - Continuous Outcome and the Integration Point: Analysis - Continuous Outcome. Click the links for setup instructions, variable details, and additional information about the integration points.
- Study objective: Two Arm Confirmatory
- Number of endpoints: Single Endpoint
- Endpoint type: Binary Outcome
- Task: Explore
Note: This example is compatible with both Fixed Sample and Group Sequential statistical designs. The R code automatically detects whether interim look information (LookInfo) is available and adjusts the analysis parameters accordingly.
The following example does not show how to load Phase 2 data into a Phase 3 trial. Please see the Consecutive Studies, Binary Outcome example for more information and step-by-step instructions.
Introduction
The intent of the following example is to demonstrate the computation of Bayesian assurance, or probability of success, and conditional assurance in consecutive studies by using custom R scripts for the Response (Patient Simulation) and the Analysis integration points of East Horizon. It features a sequential design involving a Phase 2 trial followed by a Phase 3 trial, both with continuous outcomes.
The objective is to understand how conducting a Phase 2 study can reduce the risk associated with the Phase 3 trial. This de-risking is evaluated by comparing two scenarios: (1) the probability of a No-Go decision in Phase 3 if Phase 2 is skipped, and (2) the probability of a No-Go in Phase 3 if a preceding Phase 2 trial is conducted and yields a Go decision. Specifically, we compute the conditional assurance of Phase 3 given success in Phase 2.
The scenarios covered are as follows:
- Two consecutive studies: phase 2 with a normal endpoint followed by phase 3, also with a normal endpoint.
Once CyneRgy is installed, you can load this example in RStudio with the following commands:
CyneRgy::RunExample( "ConsecutiveStudiesContinuous" )Running the command above will load the RStudio project in RStudio.
In the R directory of this example you will find the R files used in the examples:
- SimulatePatientOutcomeNormalAssurance.R - Functions to simulate patient outcomes under a normal distribution informed by mixture priors.
- AnalyzeUsingBayesianNormals.R - Implements Bayesian analysis of simulated outcomes using normal priors.
Note: This example builds on the Bayesian Assurance, Continuous Outcome example, and utilizes the same R code. Please refer to it for more information. The difference is that we now have two different studies: Phase 3 is conducted only if Phase 2 results in a Go decision.
Phase 2 Study
This study considers a two-arm fixed sample design with normally distributed outcomes , with 80 patients per treatment arm.
The figure below illustrates where this phase fits within the R integration points of Cytel products, accompanied by flowcharts outlining the general steps performed by the R code.
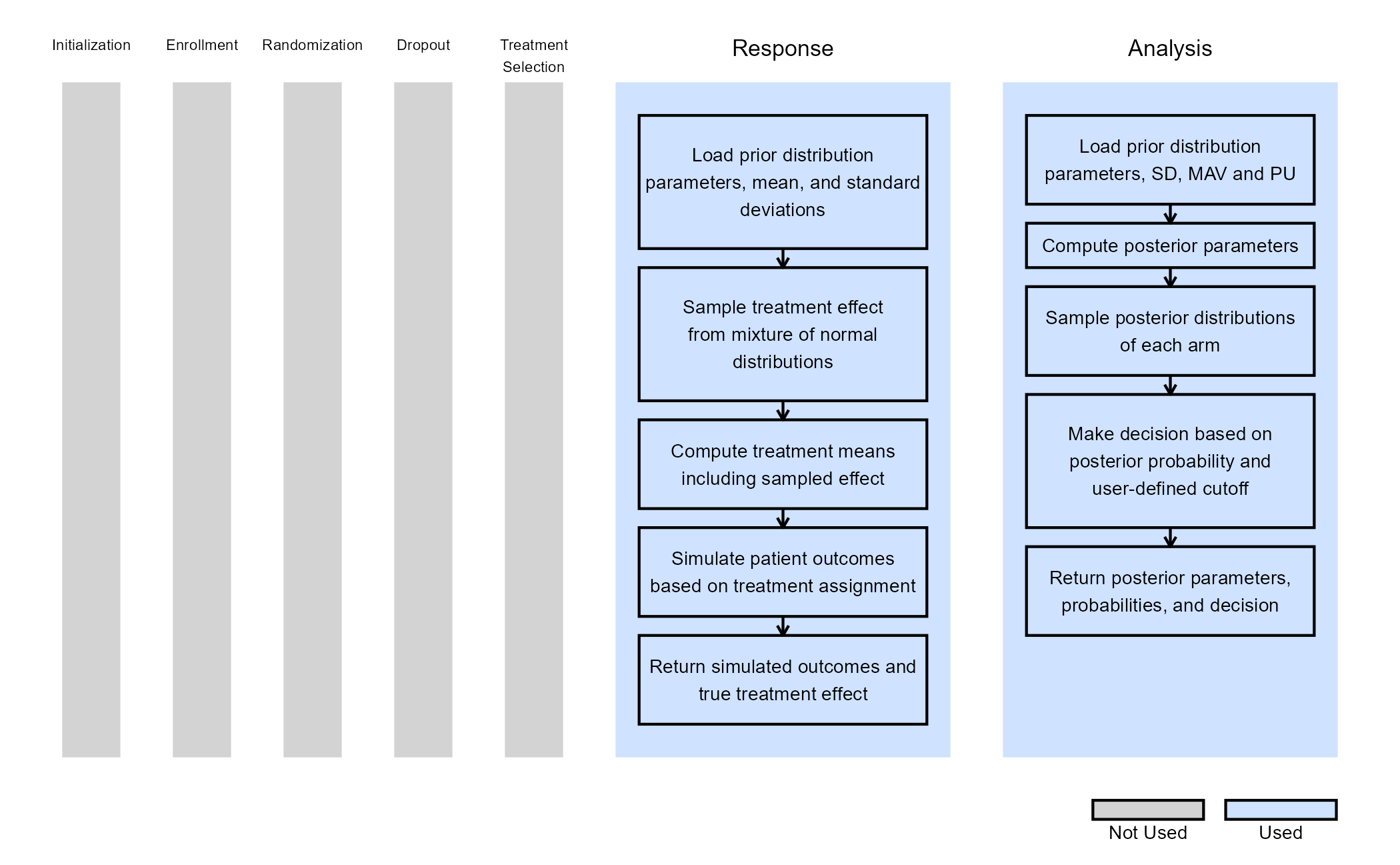
Response (Patient Simulation) Integration Point
This endpoint is related to this R file: SimulatePatientOutcomeNormalAssurance.R
This function simulates patient-level outcomes within a Bayesian assurance framework, using a two-component mixture prior to reflect uncertainty about the true treatment effect. It allows for assessing the probability of trial success (assurance) under uncertainty about the true treatment effect. Information generated from this simulation will be used later for the Analysis Integration Point.
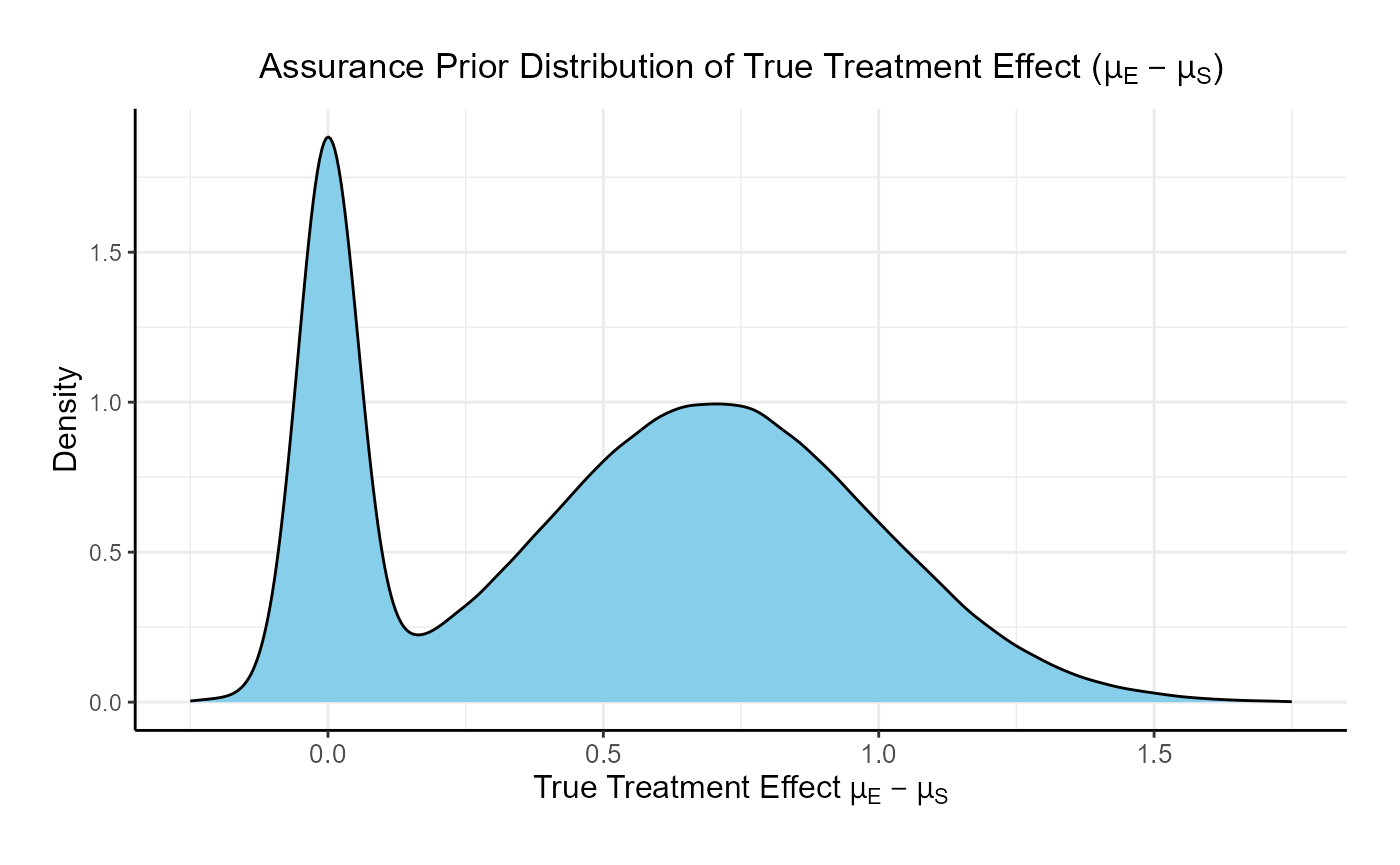
In this example, the true treatment effect () is sampled from a mixture of two normal priors:
- 25% weight on .
- 75% weight on .
The control mean is fixed, while the experimental mean is computed as . Each patient’s response is then simulated from a normal distribution corresponding to their assigned treatment group, with standard deviations defined separately for each arm.
Refer to the table below for the definitions and values of the user-defined parameters used in this example.
| User parameter | Definition | Value |
|---|---|---|
| dWeight1 | Weight of prior component 1. | 0.25 |
| dWeight2 | Weight of prior component 2. | 0.75 |
| dMean1 | Mean of prior component 1. | 0 |
| dMean2 | Mean of prior component 2. | 0.7 |
| dSD1 | Standard deviation of prior component 1. | 0.05 |
| dSD2 | Standard deviation of prior component 2. | 0.3 |
| dMeanCtrl | Mean of control arm (experimental arm will be sampled). | 0 |
| dSDCtrl | Standard deviation for control arm. | 1.9 |
| dSDExp | Standard deviation for experimental arm. | 1.9 |
Analysis Integration Point
This endpoint is related to this R file: AnalyzeUsingBayesianNormals.R
This function evaluates the posterior probability that the experimental treatment arm is better than the control arm by more than a clinically meaningful threshold, and returns a Go/No-Go decision based on a cutoff (). It uses information from the simulation that is generated by the Response element of East Horizon’s simulation, explained above.
For patients receiving treatment (S = standard/control, E = experimental), we assume the outcomes , where is an unknown mean and is a known, fixed variance.
We assume a priori that:
After observing patients on treatment , the posterior distribution of becomes:
where:
At the conclusion of the study, the posterior probability that the experimental arm exceeds the control arm by more than the minimum acceptable value (MAV) is calculated as:
The decision rule is as follows:
- If Go.
- If No-Go.
For this study, the MAV is set to 0.6 and to 0.8. Refer to the table below for all the definitions and values of the user-defined parameters used in this example.
| User parameter | Definition | Value |
|---|---|---|
| dPriorMeanCtrl | Prior mean for control arm. | 0 |
| dPriorStdDevCtrl | Prior standard deviation for control arm. | 1000 |
| dPriorMeanExp | Prior mean for experimental arm. | 0 |
| dPriorStdDevExp | Prior standard deviation for experimental arm. | 1000 |
| dSigma | Known sampling standard deviation. | 1.9 |
| dMAV | Minimum Acceptable Value (clinically meaningful difference). | 0.6 |
| dPU | Go threshold (posterior probability cutoff). | 0.8 |
Phase 3 Study
This study considers a two-arm fixed sample design with normally distributed outcomes , with 200 patients per treatment arm.
The figure below illustrates where this phase fits within the R integration points of Cytel products, accompanied by flowcharts outlining the general steps performed by the R code.
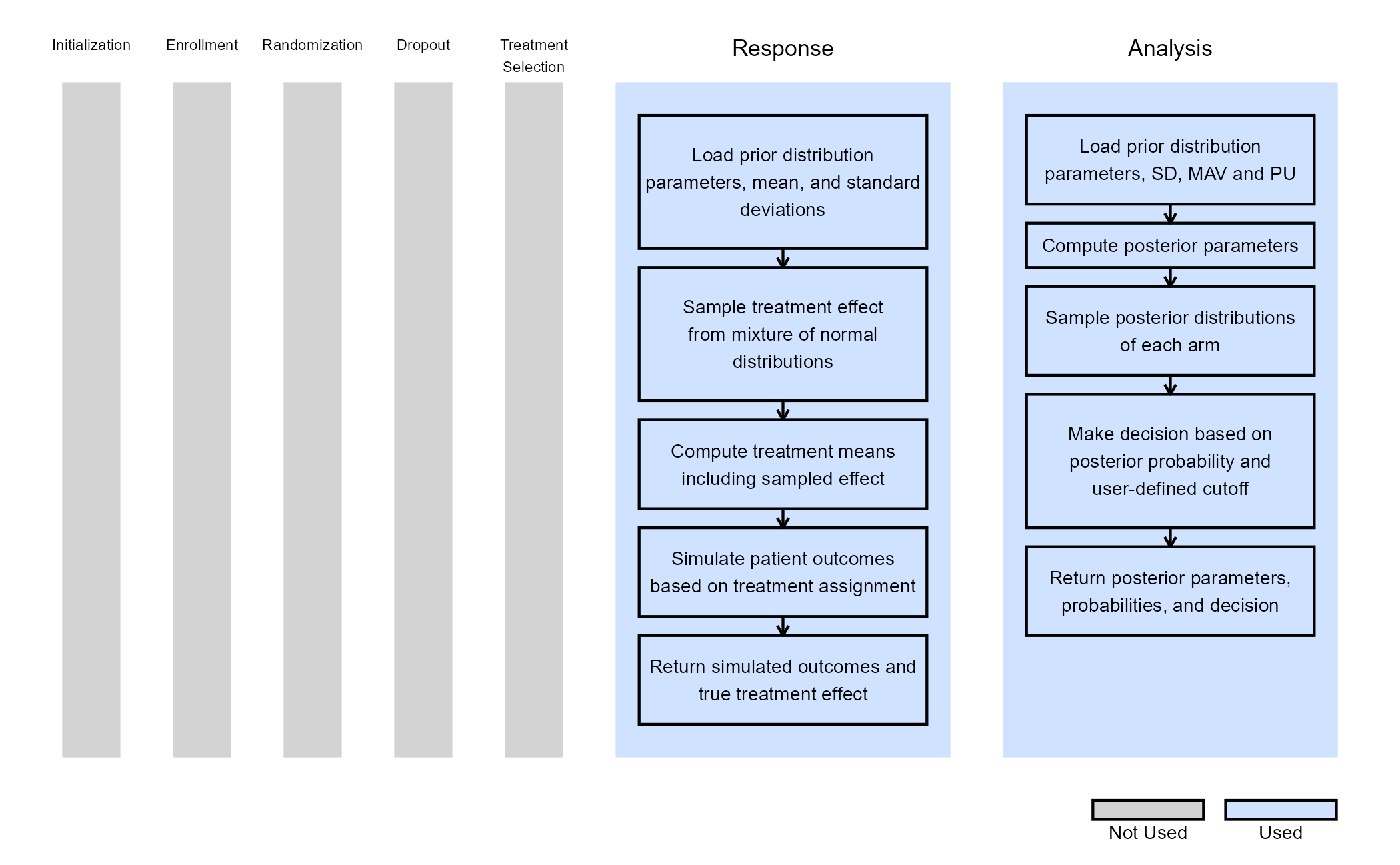
Response (Patient Simulation) Integration Point
This endpoint is related to this R file: SimulatePatientOutcomeNormalAssurance.R
The function for the Response Integration Point of the Phase 3 Study is the same as in Phase 2. Refer to the table below for the definitions and values of the user-defined parameters used in this example.
| User parameter | Definition | Value |
|---|---|---|
| dWeight1 | Weight of prior component 1. | 0.25 |
| dWeight2 | Weight of prior component 2. | 0.75 |
| dMean1 | Mean of prior component 1. | 0 |
| dMean2 | Mean of prior component 2. | 0.7 |
| dSD1 | Standard deviation of prior component 1. | 0.05 |
| dSD2 | Standard deviation of prior component 2. | 0.3 |
| dMeanCtrl | Mean of control arm (experimental arm will be sampled). | 0 |
| dSDCtrl | Standard deviation for control arm. | 1.9 |
| dSDExp | Standard deviation for experimental arm. | 1.9 |
Analysis Integration Point
This endpoint is related to this R file: AnalyzeUsingBayesianNormals.R
The function for the Analysis Integration Point of the Phase 3 Study is the same as in Phase 2.
However, for this study, the MAV is set to 0.6 and to 0.5. This design is equivalent to a t-test because the critical value would be 0.6 and having a posterior probability greater than 0.5 would indicate that the estimated treatment difference is above the critical value.
Refer to the table below for all the definitions and values of the user-defined parameters used in this example.
| User parameter | Definition | Value |
|---|---|---|
| dPriorMeanCtrl | Prior mean for control arm. | 0 |
| dPriorStdDevCtrl | Prior standard deviation for control arm. | 1000 |
| dPriorMeanExp | Prior mean for experimental arm. | 0 |
| dPriorStdDevExp | Prior standard deviation for experimental arm. | 1000 |
| dSigma | Known sampling standard deviation. | 1.9 |
| dMAV | Minimum Acceptable Value (clinically meaningful difference). | 0.6 |
| dPU | Go threshold (posterior probability cutoff). | 0.5 |
Results
For the phase 2 study independently, the probability of a Go is 26.7% and the probability of a No-Go is 73.3%.
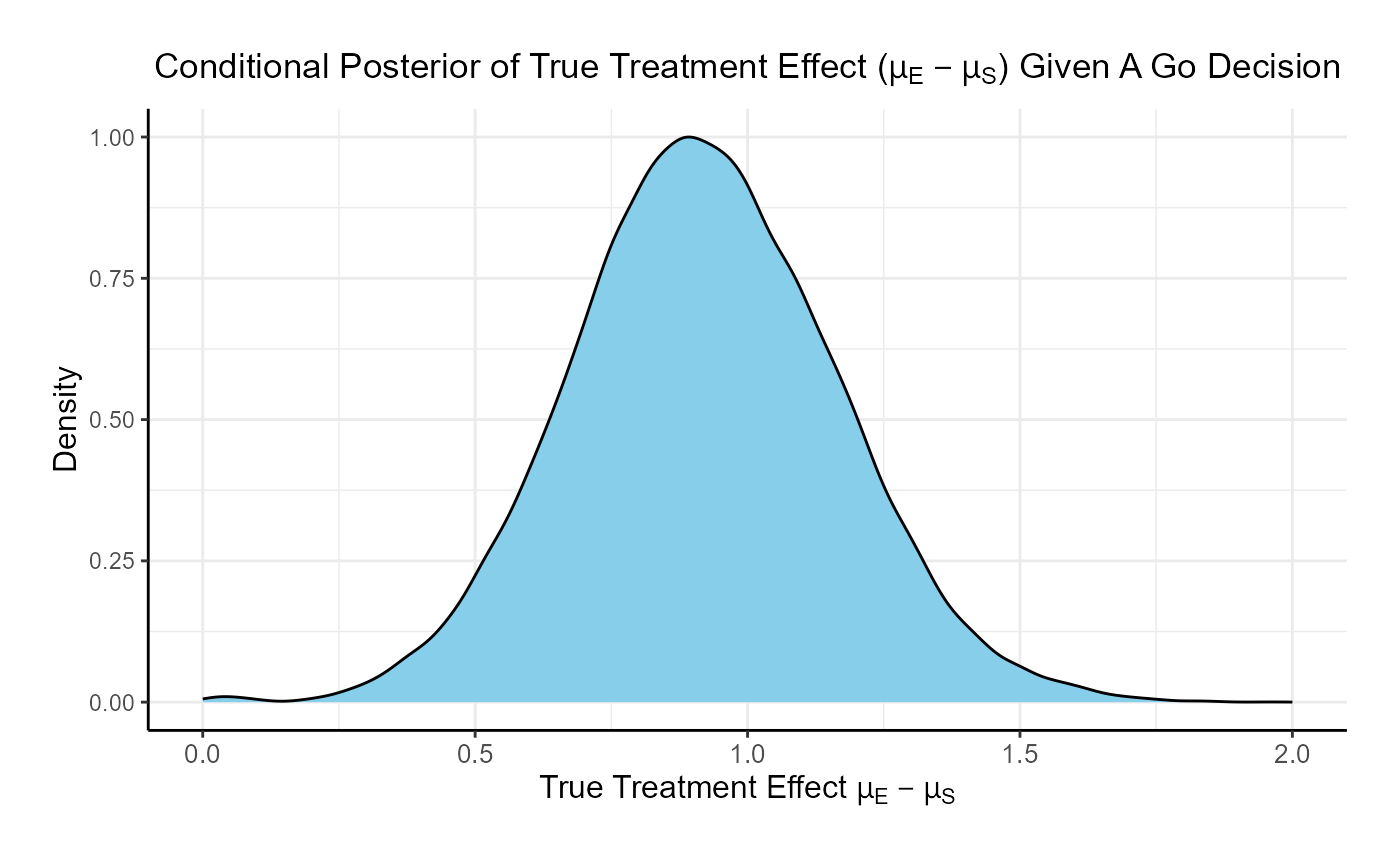
For the phase 3 study independently, the probability of a Go is 46% and the probability of a No-Go is 54%.
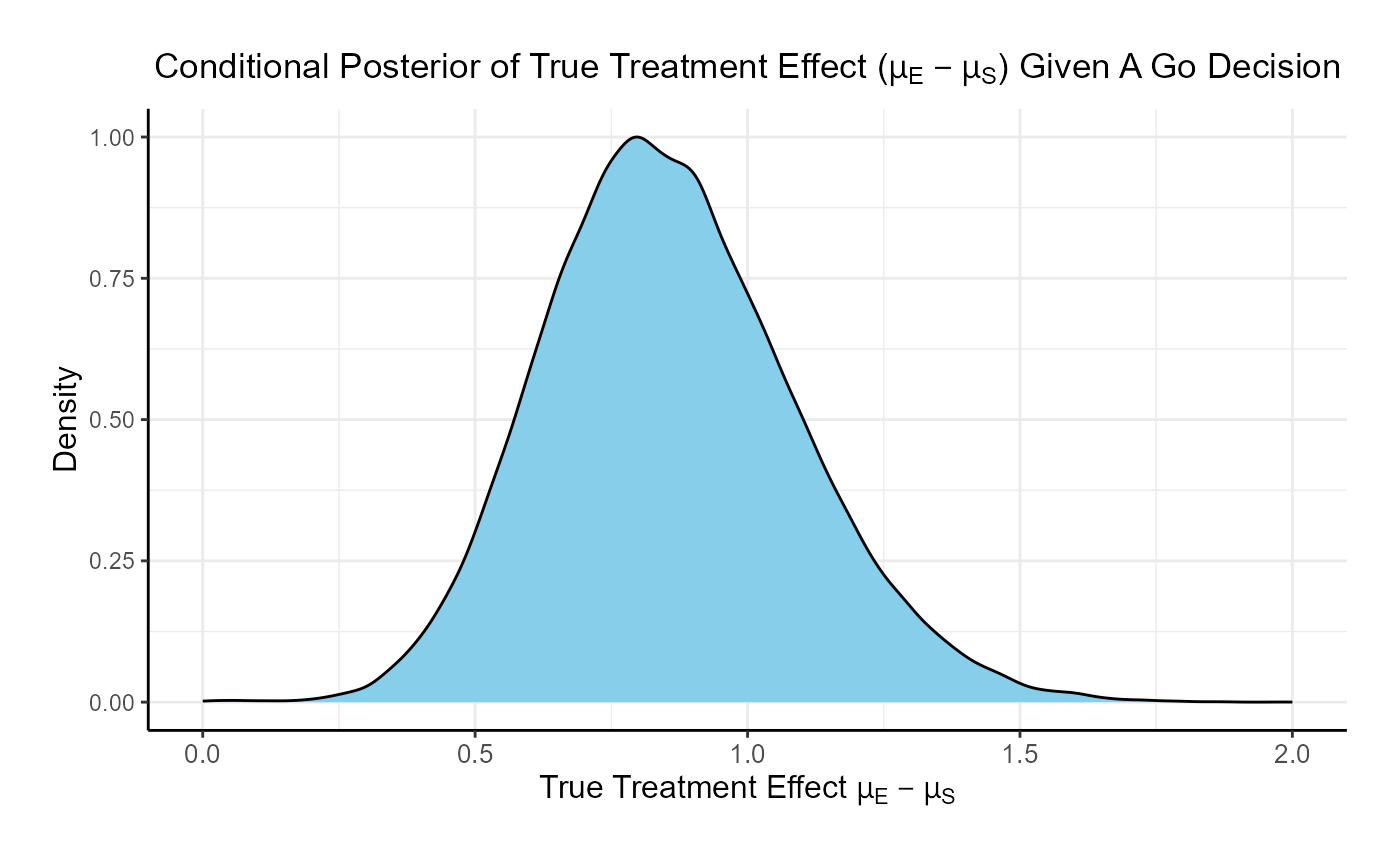
If the two studies are run sequentially, conducting Phase 3 only if Phase 2 results in a Go decision, the probability of a Go in Phase 3 is 84.8% and the probability of a No-Go is 15.2%.
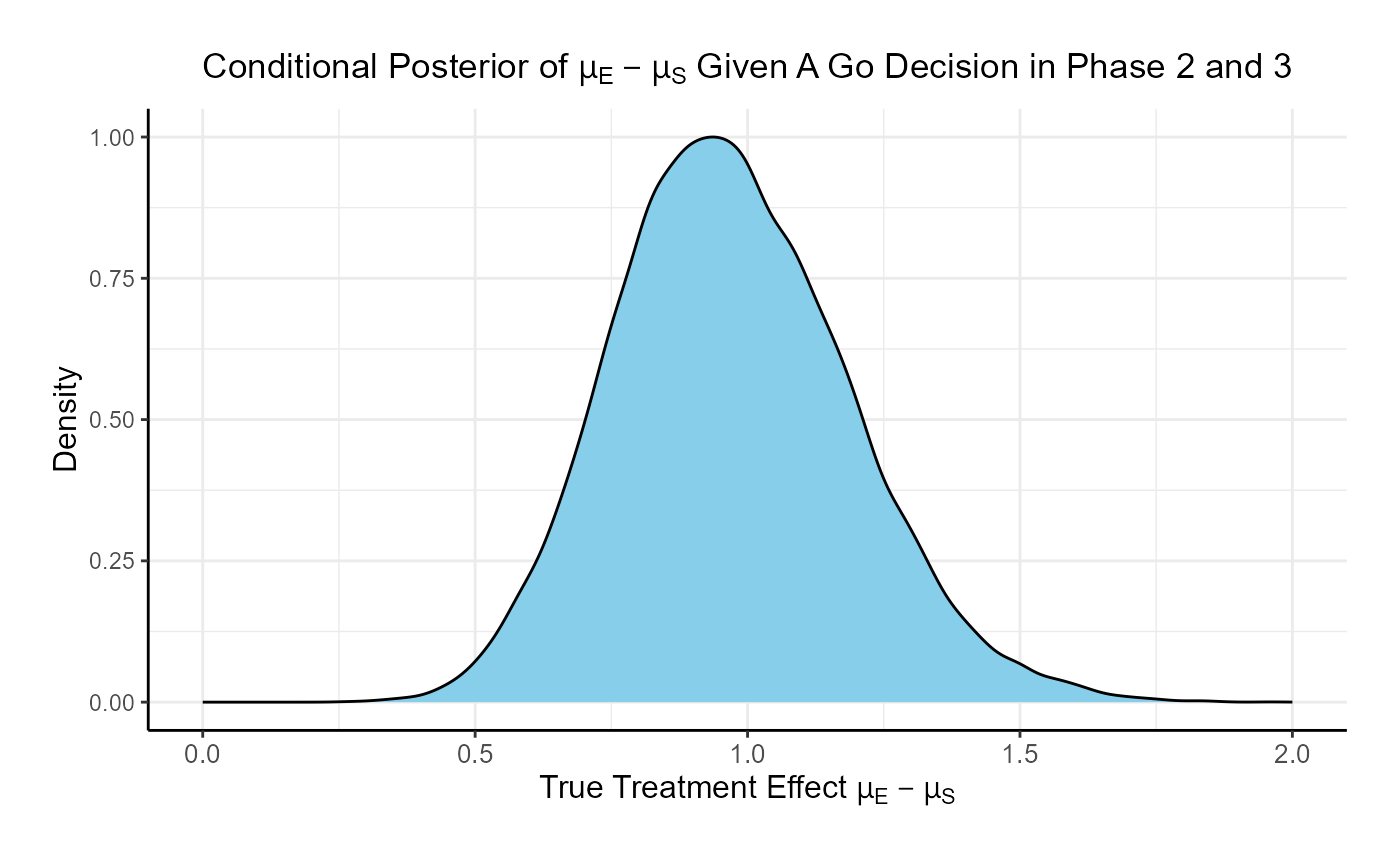
Comparing the option of running only a Phase 3 versus a Phase 2 followed by a Phase 3 if Phase 2 is successful, the probability of Go in phase 3 increases from 46% to 84.8% and the probability of a No-Go in Phase 3 decreases from 54% to 15.2%.